shelf life calculator for pharmaceutical products
Input the TAA and TRT values both in Celsius for your product. Shelf life is defined as the time period over which the concentration of the active drug ingredient in the formulation drops by 10 percent from its value at time of manufacture.

Evaluation Of Shelf Life Of Drug Products By Arrhenius Equation Part Ii Youtube
Accelerated Stability Testing And Shelf-Life Calculation.
. FDA supports a public health program involving other partners to extend the expiration dates for a limited number of carefully selected drug products. True shelf life estimated shelf life supported shelf life maximum shelf life and labeled shelf life. Click Calculate at the bottom of the.
It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. N 50-25 10 25. SHELF LIFE CALCULATION Shelf life is the period of time from the date of manufacture that a drug product is expected to remain within its approved product specification while stored.
ICH Q1E guideline provides guidance for the estimation of the shelf life of the pharmaceutical products and substances. The wall thickness and grade of the plastic depends on how. Any drug product packaged after September 29 1979 except for those specifically exempt by 211137.
For example the shelf life of a product at 50 C is 32 days. The product is stored at 400 C - 20 C with. Based on published information it.
The shelf life time t with a minimum label claim of 95 can be. A product stored for stability at or near 15 C may have quite a different quality profile at its expiration date than a product stored at or near 30 C. The International Conference on Harmonisation ICH of Technical Requirements for the Registration of Pharmaceuticals for Human Use guidance document Q1AR2 ICH Q1A.
Input the Aging Factor Q10 value for your product. Normal storage temperature is 25 C. The shelf life of a drug preparation is the amount of time that the product can be stored before it becomes unfit for use through either chemical decomposition or.
Shelf life is determined by the evaluation of whole stability data of. At that time Q10n 3 25 156. Lets imagine that you have a product manufactured on the 21st of December 2019.
The shelf life of food pharmaceuticals and other perishable items is defined with one of the factors ie permeability or flux. This can also be used to. Under the Shelf-Life Extension Program.
SLIMStat created by the makers of SLIM HA Scientific is an extremely easy-to-use software developed and validated specifically to determine the shelf life of drug products that have been. Stability Testing And Shelf Life Estimation Shelf Life Calculator For Composites And Other Materials Solved 1 The Rate Constant Of Degradation Of A Pharmaceutical Product Degrading. The default Q10 value is set at 2.
A practical example of the cosmetic expiration date. On the label you can see the following. Suppose Q10 3.
Shelf life explains duration of the medical device to be stabile to retain the sterility of the package and the device performance. Q10 value can be changed however default is Q1020 we. This document assists with establishing the expiration period of a production bath of a medicinal.
This guideline applies to human and veterinary medicines. November 09 2021 0. By rule of the thumb most of my pharma marketing friends will agree if one cannot have a product life of at least 18 to 24 months better to not market such products.
Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of. The five new terms that are necessary for a coherent discussion of shelf life are. Full model product shelf life 378 200 250 pharmaceutical stability shelf life august 1 2010 21 16 141 35 58 77 88 23 frequency 0 50 100 150.
Absence of expiration date. Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product.

Desiccant Calculator Superdrysim Baltimore Innovations

How To Calculate Expiration Dates In Excel
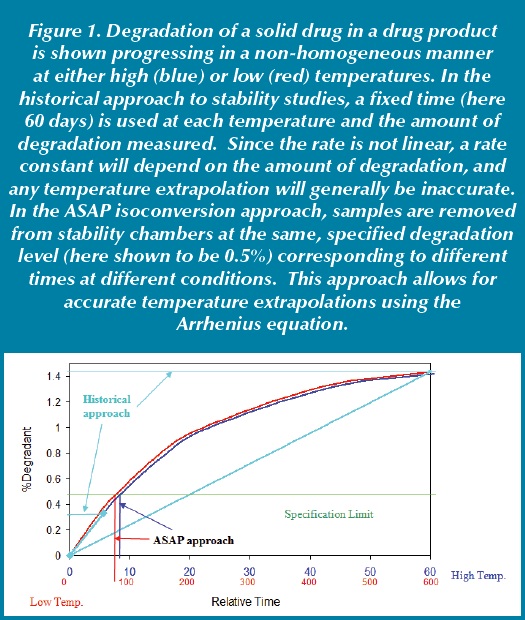
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

How To Calculate Expiration Dates In Excel
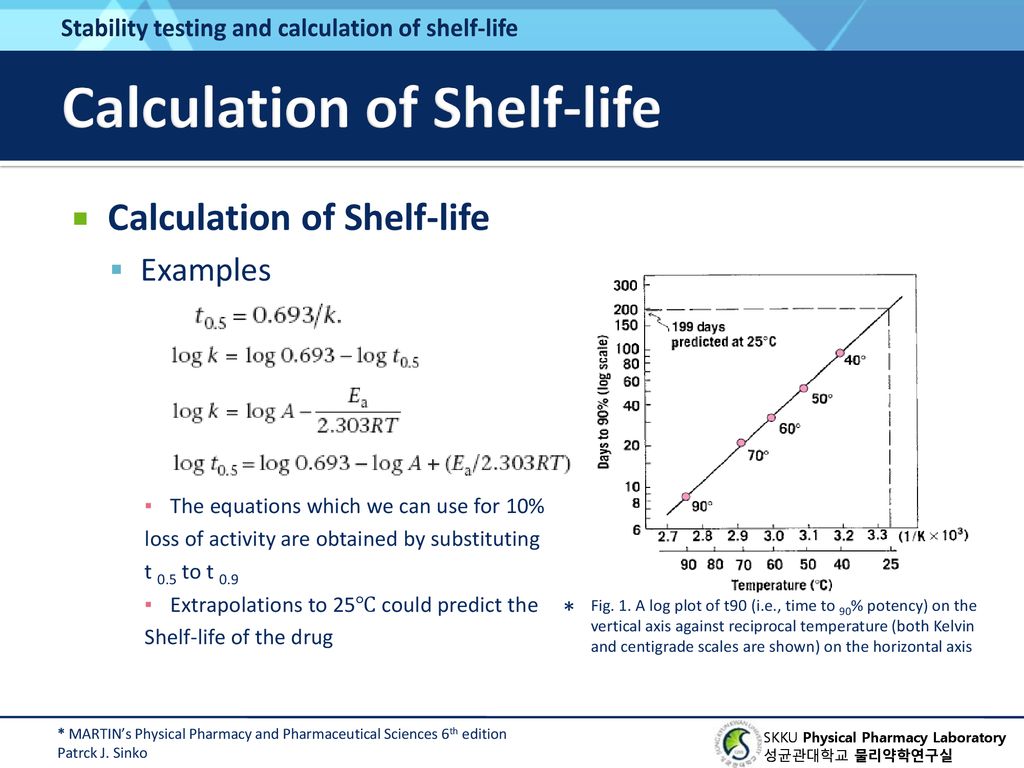
Chapter 14 Chemical Kinetics And Stability Ppt Download

Assessing Shelf Life Using Real Time And Accelerated Stability Tests

Shelf Life Determination Of Pharmaceutical Products

Can Anyone Help With The Calculation Of Shelf Life And Half Life Period Of Any Protein Researchgate

Checking And Calculating The Shelf Life Expiration Date Sap Help Portal

Shelf Life Calculation Of Drugs
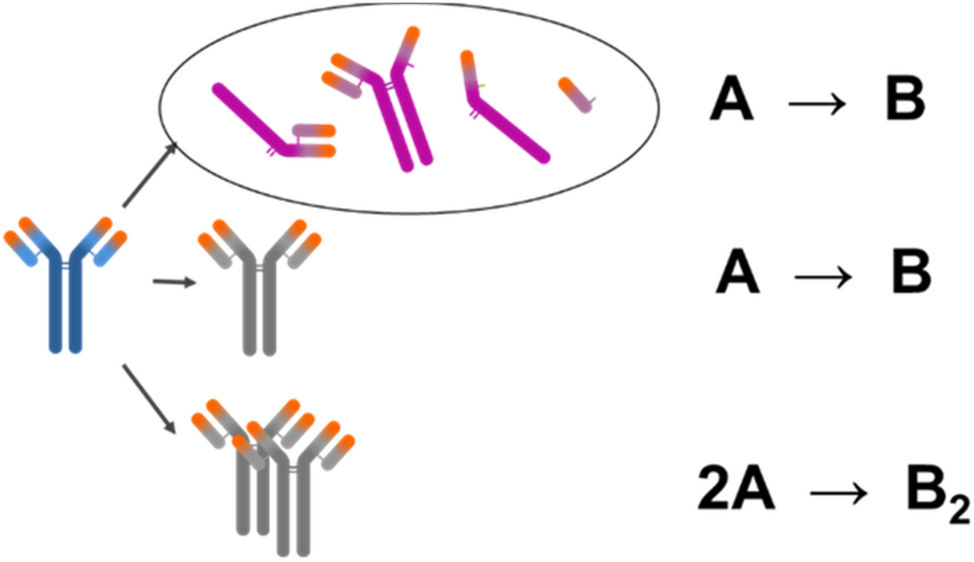
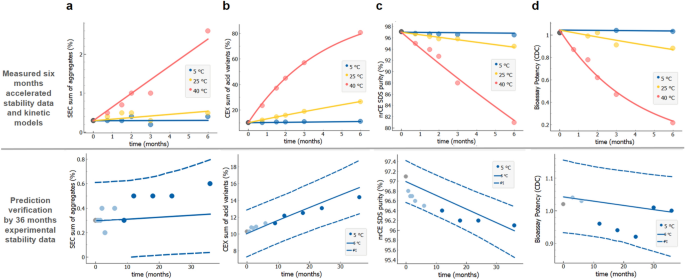
Long Term Stability Predictions Of Therapeutic Monoclonal Antibodies In Solution Using Arrhenius Based Kinetics Scientific Reports
What Is The Real Shelf Life Of Drugs I E They Are Still Effective Or At Least Safe Quora

Estimation Of Shelf Life Degradation In Days 1 St Condition First Download Scientific Diagram
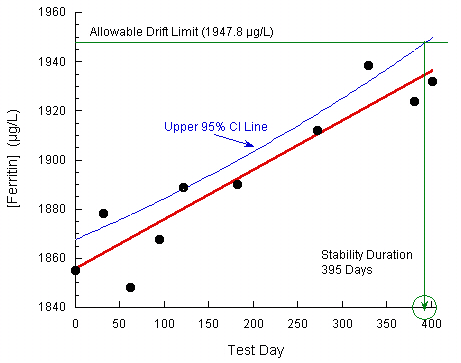
Stability Testing And Clsi Ep25 A Westgard

Why Do We Need To Batch Produce And Use Batch Numbers

Shelf Life Calculation Of Drugs

Pdf Arrhenius Equation Modeling For The Shelf Life Prediction Of Tomato Paste Containing A Natural Preservative Shelf Life Prediction Of Tomato Paste